Hepcidin reference material
Secondary reference material for standardization of hepcidin assays
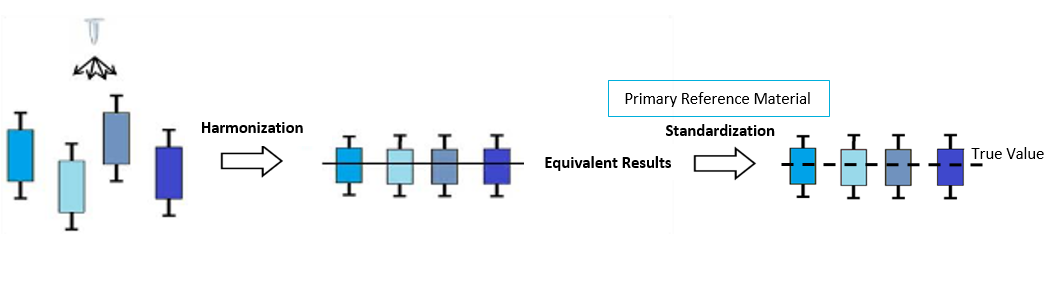
Currently, hepcidin levels differ between labs and assays. An important step toward the international use of hepcidin in both diagnostics and research is standardization of hepcidin assays worldwide. Consensus among hepcidin results for various methods is necessary to facilitate interpretation of clinical studies and future implementation of assays in the clinic. This will enable the establishment of uniform reference intervals and decision limits, as results are comparable and traceable to SI units.
Equivalence between results can be improved by the appliance of a commutable secondary reference material (sRM), developed by Hepcidinanalysis.com.1,2 The sRM is based on the principle of three-point calibration, and therefore consists of three lyophilized samples. These samples cover the low, middle, and high concentrations of the hepcidin reference range in order to increase accuracy. Initially, and two-point calibrator was introduced that covered the low and middle concentration ranges, but a third was recently developed and showed improved accuracy of calibration at higher concentrations3. All calibrators are produced following technical procedures to achieve efficient harmonization, as recently developed by the International Consortium for Harmonization of Clinical Laboratory Results (ICHCLR).4
A primary reference material (pRM) was developed by the Laboratoire National de métrologie et d’Essais (LNE RM hepcidin 501a), based in Paris, France. Using this RM we assigned a value to our three-leveled RM with an isotope dilution mass spectrometry-based candidate reference method. Wordwide implementation of this RM to calibrate analytically reliable methods, including all laboratories and manufacturers of diagnostic kits, will allow standardization of hepcidin assays with results traceable to SI units.
Calibration will benefit the clinical, pharmaceutical and research communities by ensuring comparable results, uniform reference values and clinical decision limits.
For more information or in case of interest in purchasing the material, please send an e-mail to hepcidinanalysis@radboudumc.nl
References
- van der Vorm LN, Hendriks JC, Laarakkers CM, et al. Toward Worldwide Hepcidin Assay Harmonization: Identification of a Commutable Secondary Reference Material. Clin Chem. 2016; 62(7): 993-1001.
- Diepeveen LE, Laarakkers CMM, Martos G, Pawlak ME, Uğuz FF, Verberne KESA, van Swelm RPL, Klaver S, de Haan AFJ, Pitts KR, Bansal SS, Abbas IM, Fillet M, Lefebvre T, Geurts-Moespot AJ, Girelli D, Castagna A, Herkert M, Itkonen O, Olbina G, Tomosugi N, Westerman ME, Delatour V, Weykamp CW, Swinkels DW. Provisional standardization of hepcidin assays: creating a traceability chain with a primary reference material, candidate reference method and a commutable secondary reference material. Clin Chem Lab Med. 2019;57(6):864-872.
- Aune ET, Diepeveen LE, Laarakkers CM, Klaver S, et al. Optimizing hepcidin measurement with a proficiency test framework and standardization improvement. Clin Chem Lab Med. 2020;59(2):315-323.
- Myers GL, Miller WG. The International Consortium for Harmonization of Clinical Laboratory Results (ICHCLR) – A Pathway for Harmonization. EJIFCC. 2017; 27(1): 30–36.
